美國 FDA 認可活髮產品
LaserCap ( USA ) 激光活髮帽 - 香港獨家分銷
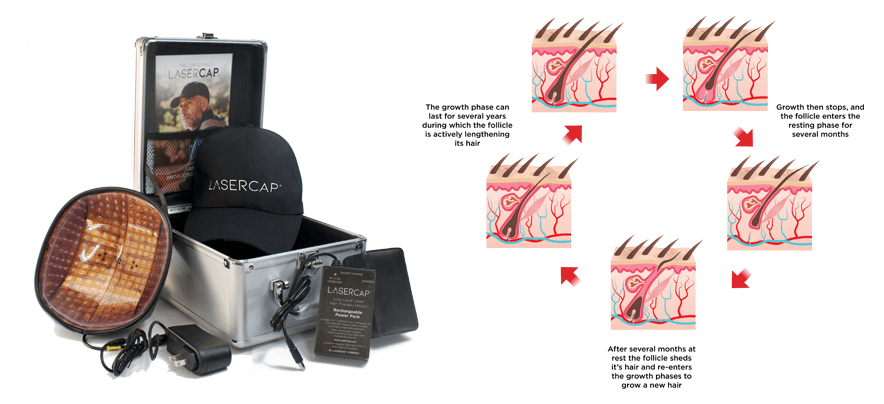
我們的 LaserCap 使用低能量激光( LLLT ),激活休止期的毛囊,這是一種安全物理性的療法,經科學證明可促進男女毛髮再生。 LaserCap 非常易於使用。給電池充電接駁後,將 LaserCap 套進您喜歡的帽子中,再將帽子放在稀疏的頭皮上,照射 20-30 分鐘。便攜式的 LaserCap 可在您自己的家中,健身房,日常活動的任何地方,任何時間使用!每隔一天使用一次,便即可使頭髮再生並恢復自然髮質。
FDA 510(k)認可
每年都有成千上萬的醫療器材推出市場,全都聲稱它們具有案例研究和醫療實證。但是,其中許多都衹是誇大性的宣傳。為了避兔市民誤用危險的醫療用品,美國食品和藥物安全局 FDA 要求生產商進行 510 (k) 的嚴格測試和證明,測試包括臨床研究和實驗室數據搜集,經過專家評審必須滿足特定安全要求,才能批准將其出售給公眾。在選購任何激光生髮產品時,應先確認已獲得 FDA 510 (k) 許可。
LaserCap 美國總公司網頁
激光活髮的科學原理
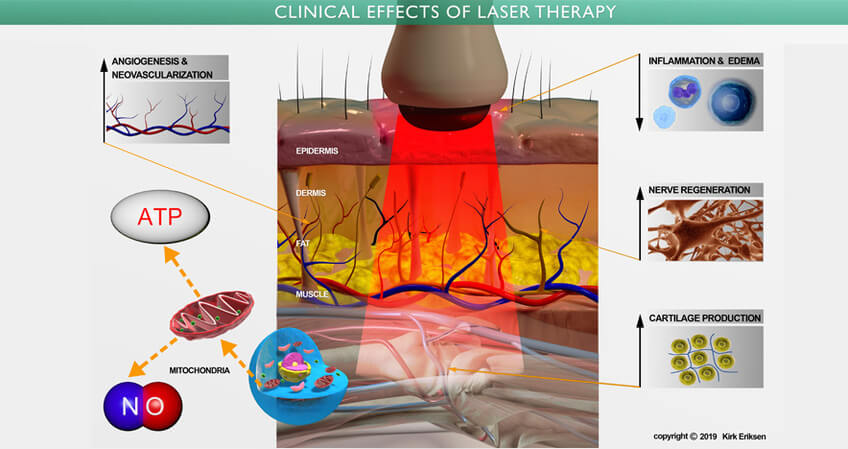
低能量激光療法( Low Level Laser Therapy, 簡稱 LLLT ),也稱為冷激光療法,光生物調節,生物刺激和光療,已在數千篇同行評審的出版物中表明,它們可以增加細胞存活,增殖和功能。 被細胞線粒體吸收後的激光產生以下作用
• 刺激細胞的正常生長和功能:
• 合成蛋白,增加細胞代謝
• 調動體內的鈣離子
• 增強 ATP 的產生
• 促進皮下血液的微循環
• 允許細胞吸收更多的營養
• 增加細胞有氧呼吸
• 通過活性氧,激活 「 誘導轉錄因子 」
問題不再是光是否具有生物作用,而是治療性激光,和 LED 的能量,如何在細胞和生物體水平上起作用,以及這些光源的不同用途的最佳光參數是什麼。
雙向劑量反應
在細胞培養、動物測試、和臨床研究中的多項研究,已證明的一個重要觀點,是相向劑量的相反作用。LLLT 之所以稱為 「 低能量激光 」,是相對於更高頻率及功率的激光而言,當激光的光劑量低於某值,會有生髮作用相反,若光劑量大於某值時,不但降低治療脫髮的效果,更高劑量的激光甚於導致陰性結果破壞毛囊。所以激光可以生髮,亦可以脫毛,要視乎光劑量的高低。
傳遞方式
傳遞治療光的方法多種多樣,要根據不同參數( 波長、輸出功率、連續波或脈衝操作模式、脈衝參數、偏振態等 )和各種光源( 激光,LED )的使用來決定。 2002 年 MicroLight Corp. 獲得了 ML 830nm 二極管激光治療腕管綜合症的 510K FDA 許可。有幾項對照試驗報告患者疼痛明顯改善,客觀結果指標有所改善。現今在臨床研究下,已經有幾種光源用於用於治療各種肌肉骨骼疾病。
改善脫髮
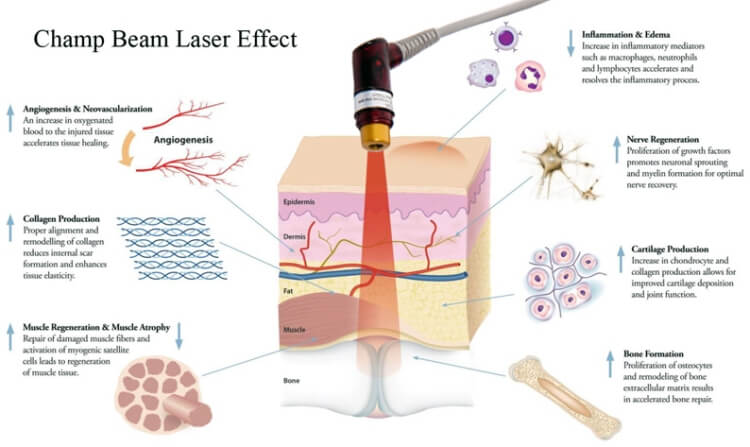
已證明激光通過刺激毛囊,可產生以下效果:
• 有效地維持毛囊細胞的正常生長和功能
• 增加細胞代謝,速進毛囊的血液循環
• 增加毛囊細胞的存活,增殖和功能
• 刺激皮脂腺讓頭髮更亮麗
• 增加毛囊的黑色素分泌讓頭髮更
• 加快毛髮的再生長速度。
臨床對照試驗顯示,激光能夠改善雄激素性脫髮、及其他類型的脫髮。已有餘千的出版刊物,作出此類報導。2009 年美國藥物管理局 FDA 經業界評議研究,決定批准激光合法成為激發頭髮重新生長的產品。壞死或嚴重萎縮的毛囊,在脫髮初期進行光療,能顯著改善頭髮的密度和質量。 一般男士或女士脫髮均可適用。更可用與植髮,藥物治療一并使用,得到更顯著的改善。
植髮康復期的使用

植髮最難過的並非手術過程,而是等待頭髮生長的漫長日子。毛囊從捐髮區取出,移植到禿髮的部位,需要至少一星期才會重生接駁血管,加上植入毛囊會給頭皮造成創傷,引起腫脹,做成短暫性的缺氧。
移植的毛囊細胞為了要適應新環境,會逼使進入休止期,所以九成之上移植的毛囊髮梢,會於手術後六個星期內䬰脫落,這種暫時性的脫髮被稱為 Shock Loss,要六至八個月內再重新長出。雖然這是正常的反應,但很多病人會非常擔心,渴望頭髮會快些生長出來。經過多年的臨床實證,發現於植髮後使用低能量激光,有以下好處:
• 能加速植髮區的傷口癒合,減少手術後腫脹,發紅和發炎
• 減少結痂,通常植髮後第四天大部份的痂都已脫落
• 增加了照射部位的血液供應,減少脫髮 Shock Loss
• 有助縮短移植毛囊的休止期,頭髮更迅速地長出,提高毛囊的存活率
毛囊細胞生長能源 ATP
三磷酸腺苷 ATP 是毛囊細胞生長所必需的能量來源,移植或受創的毛囊,更需要充足的 ATP 能量供應。激光頭髮療法,可刺激細胞中的線粒體 Mitochondria ,從而增加 ATP 的產生,Sunetic 20 分鐘的治療,能夠使毛囊細胞的線粒體恢復活力,透過提高存活率,令其多移植的毛囊最終能長出健康的永久性頭髮。
低能量激光的其他應用
促進傷口癒合
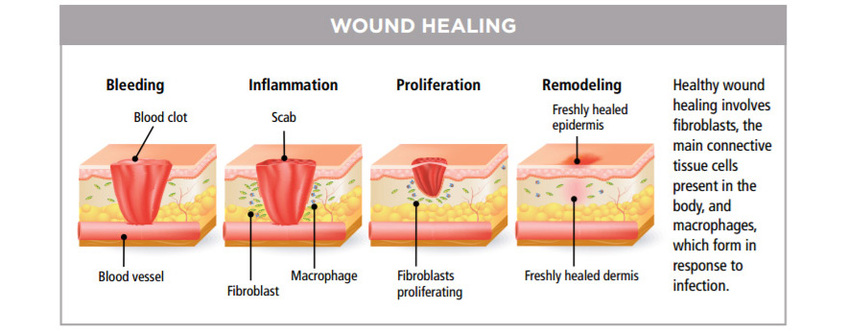
自 1960 年起,LLLT已普遍用於治療慢性潰瘍,慢性疼痛,頭痛、肌肉骨骼及神經痛症且不會產生不良副作用。激光的光波被細胞的線粒體 Mitochondria 吸收後,會增加細胞呼吸,並通過活性氧的誘導,激活細胞核的轉錄因子的,能減低痛症,發炎與紅腫,使傷口的深層細胞組織及神經,加速康復,並防止細胞受損。這些療法已在餘千的出版刊物、廣泛流傳。
激光活髮的歷史演變
1967
光療法是人類使用的最古老的治療方法之一( 歷史上被埃及人稱為太陽療法 )。 在發明首部個激光儀器的數年後的 1967 年,匈牙利塞梅爾魏斯恩大學 ( Semmelweis University, Budapest, Hungary ) 的一位學生「 布達佩斯 」 Endre Mester,使用激光照射白老鼠,來研究測試激光會否引起小鼠癌症。他首先把白老鼠背部的毛剃掉,然後把他們分成兩組,其中一組用低能量的紅寶石激光 ( 波長694nm ) 給牠們照射,另一組作對照。
結果被激光照射的老鼠,沒有患上癌病,反而背部的毛生長得還要被對照組還要快。 這就是第一個證明激光能刺激生髮的實例。多年來激光在臨床上應用於治療中風、促進傷口癒合、對骨科疾病和慢性炎症等,有緩解止痛功效。也有效地而改善脊髓損傷,周圍性神經退化,心臟病,退化性腦部疾病、和創傷性腦損傷等。
1989
有人提出,激光在細胞水平上的作用,是基於細胞呼吸鏈組分對光波的吸收。細胞呼吸發生在稱為線粒體 Mitochondria 的亞細胞中。線粒體內膜包含 5 個完整膜蛋白複合物: NADH 脫氫酶( 複合物I )、珀酸脫氫酶、細胞色素 c 還原酶、細胞色素 c 氧化酶、ATP 合酶、和兩個自由擴散分子泛醌和細胞色素 c,當電子從一個複合體傳遞到下一個複合體時,呼吸鏈會釋放能量,其作用類似一個微型電池。之後呼吸鏈會重新進入線粒體 「 儲電 」 ,不斷重覆以上動作。
1995
對五個作用譜的分析表明,動物細胞的主要光感受器是細胞色素 C 氧化酶,該酶包含兩個鐵中心,血紅素 a 和血紅素 a3 ,以及兩個銅中心 CuA 和 CuB 。完全氧化的細胞色素 C 氧化酶具有 Fe(III)氧化態的鐵原子和 Cu(II)氧化態的銅原子,而完全還原的細胞色素 C 氧化酶具有的 Fe(II) 態鐵和 Cu(I)銅)氧化態和甲酸酯。該酶的所有許多單獨的氧化態都有不同的吸收光譜,因而解釋了 LLLT 作用光譜的細微差異。
2009
美國食品和藥物安全局 FDA 在 2009 年,決定批准激光合法成為激發頭髮重新生長的產品。多項低能量激光產品包括 Capillus, Hairmax, Sunetics, LaserCap ... 等亦先後已通過 FDA 的驗証,用作改善成年男性和女性的遺傳性脫髮 (Androgenetic Alopecia),促進毛囊再生。
最近的發展
Karu 小組的最新論文給出了 LLLT 作用譜中的四個波長:
1) 613.5 – 623.5 nm, 2) 667.5 – 683.7 nm, 3) 750.7 – 772.3 nm, 4) 812.5 – 846.0 nm. 分子吸收光子會導致電子激發態,因此會加速電子轉移反應。 更多的電子傳輸必然導致 ATP 的產量增加。
光誘導的 ATP 合成增加和質子梯度增加導致 Na+ / H+ 和 Ca2+ / Na+ 反轉運蛋白,以及所有 ATP 驅動的離子載體 ( 例如 Na+ / K+ ATPase 和 Ca2+ 泵 )的活性增加。 ATP 是腺苷酸環化酶的底物,因此 ATP 水平控制著 cAMP 的水平。 Ca2+ 和 cAMP 都是非常重要的第二信使。 Ca2+ 尤其能調節人體的幾乎所有過程 ( 肌肉收縮,凝血,神經信號傳遞,基因表達等 )。
激光生髮療法學術著作
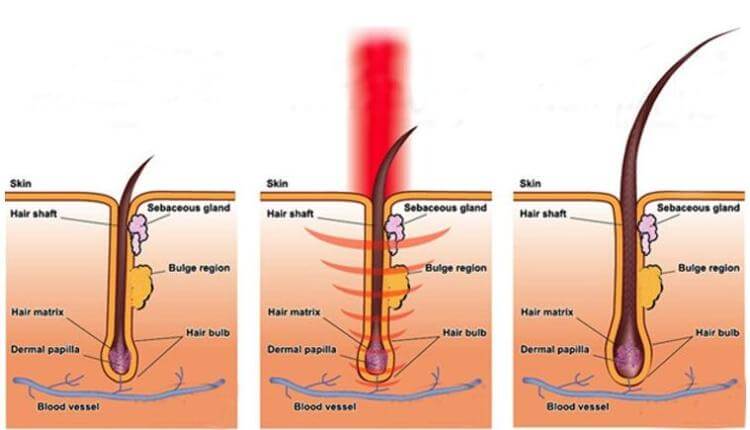
激光生髮療法, 也被稱為低能量激光療法、光療、冷激光治療,光生物調整,生物刺激及光線療法,這些療法已在餘千的出版刊物、廣泛流傳、證明了紅激光或低能量激光能有效地增加細胞的存活,增殖和功能。紅激光的光波被細胞的線粒體 mitochondria 吸收後,會增加細胞呼吸,並通過活性氧的誘導,激活細胞核的轉錄因子。以下收錄光學專家 Dr Hamblin 的一篇著作。
Scientific Study - Low Level Laser Therapy for Hair Growth, Dr Hamblin 2009
自從 Mester 的第一個開創性出版物,報導了刺激小鼠毛髮生長以來,幾乎沒有關於動物模型中的 LLLT 刺激毛髮生長的後續研究。Mester 的研究涉及每週從 694nm 的紅寶石激光器向黑色 C57 和白色 Balb/c 小鼠的脫毛腹部區域傳送 1 Joule 脈衝光 ( 1 毫秒脈衝持續時間 ) 到 1cm2 點,長達 11 週。在每次相繼治療之前,再次將皮膚脫毛。
在第 5 次和第 7 次治療之間,在所有黑色動物中,均觀察到了受輻照斑點生長中毛髮生長的增加。該反應一直持續到第 9 次治療,其頭髮生長強度的特徵是,在每次照射時完全裸露的地方,僅在照射後 4-6 天觀察到與其他身體部位一樣濃密的毛髮生長。另一方面,發現在第 9 次輻照之後,僅在輻照部位頭髮生長停止了。取而代之的是,在受輻照區域周圍觀察到了環形毛髮的生長。
這種環形毛髮生長首先出現在動物上,首先觀察到中央毛髮生長刺激。在第 7 次和第 9 次照射之間,所有處理過的黑色小鼠的外周生長均出現,強度隨小鼠而變化。在白色小鼠中,直到第 8 次輻照都未檢測到對毛髮生長的影響。所述的黑色小鼠的中央生長僅在第 8 次照射後才開始形成。
進一步的照射導致了某些小鼠中所述的毛髮生長,但是在某些小鼠中已經出現了第二期的周圍毛髮生長特徵。對照動物的毛髮生長如下:脫毛的皮膚使頭髮緩慢而分散地生長。但是,在一半的對照動物中(在黑白小鼠中),均未觀察到進一步的毛髮生長。同時,某些動物出現了散亂的毛髮生長,但在另一些動物中卻出現了不典型的,有時是對角線的條紋。
儘管 LLLT 設備已經廣泛銷售並用於頭髮再生,但只有少數文獻,報導包含一些觀察到 LLLT 引起的患者頭髮生長以及改善或治療任何類型的脫髮的觀察結果。一個日本小組報導了關於使用 Super Lizer ( 一種線性偏振光源,提供 1.8W 的 600–1600nm 光波 )來治療斑禿。與未經治療的病灶相比,每 1 或 2 週 3 分鐘的療程產生明顯的毛髮生長,佔 47% 的患者。
一個西班牙小組報告關於使用氦氖激光器治療脫髮性雄激素和斑禿。芬蘭的一份報告比較了三種用於男性型禿髮的光源( HeNe 激光、670 nm 的 InGaAl 二極管激光、和非相干的 635nm LED ),並測量了頭皮的血流量。
最近的工作,發現了一些參與頭髮生長調節的生物學機制,這些機制可能是解釋 LLLT 刺激作用的良好候選者。 Peters 等發現神經生長因子 ( NGF ) 通過其高親和力受體 ( TrkA ) 促進增殖。並確定 NGF 和 p75 是重要的頭髮生長終止劑。通過 rtPCR,我們發現,鼠背皮膚中 NGF/proNGF mRNA 水平,在生長期初期達到高峰,而在生長期中 NGF/proNGF 蛋白水平達到峰值,表明生長期中的高周轉率,和生長期中的蛋白質積累。
通過免疫組織化學,在整個週期中,在表皮和毛囊的增生區室中發現了 NGF 和 TrkA。包含 NGF 和 proNGF 的商業 7S NGF 可以促進器官培養的早期生長期、小鼠皮膚中的生長期發育,同時還可以促進後期生長期皮膚中的生長期發育。因此,數據提示了NGF/TrkA 的生長期促進及支持作用。
該小組的另一份報告研究了p75 神經營養蛋白受體 ( p75NTR ) 的表達和功能,該蛋白與鼠皮膚自發性催化發展中的細胞凋亡控制有關。他們發現,p75NTR 單獨在退化的外根鞘的 TUNEL+/Bcl2- 角質形成細胞中強烈表達,而 p75NTR 和 TrkB 和/或 TrkC 均由非退化的 TUNEL-/Bcl2+ 繼發性發芽角質,形成細胞表達。與野生型對照相比,p75NTR 基因敲除小鼠具有明顯的致癌性遲緩。
取而代之的是,過表達 NGF 的轉基因小鼠( 啟動子:K14 ), 表現出明顯的催化作用。Schwartz 等人在 2002 年報導,氦/氖激光照射( 3Joule/cm2 ) 將 NGF mRNA 的水平提高了五倍,並增加了NGF 的釋放。體外培養的肌管培養基。這與肌管中細胞內鈣的瞬時升高有關。Yu 發現,培養的人類角質形成細胞釋放的神經生長因子顯著增加。因此,推測 LLLT可能通過 NGF/p75NTR 信號傳導系統影響頭髮再生。
Zcharia 將內切糖「 苷酶乙酰肝素酶 」鑑定為鼠毛生長的重要調節劑。乙酰肝素酶對硫酸乙酰肝素形成的細胞外基質屏障的降解,使細胞能夠通過細胞外屏障移動,並從細胞外基質貯庫中,釋放出生長因子,從而使其具有生物利用度。這允許濾泡幹細胞後代,遷移並重建濾泡下部,這是毛幹形成的先決條件。
酰肝素酶促進了隆起的角質形成細胞,在體外遷移穿過細胞外基質屏障的能力。在過表達乙酰肝素的轉基因小鼠中,乙酰肝素酶水平的升高,可增強活性毛髮的生長,並能在化學療法誘發的脫髮後,更快地恢復毛髮。胸腺素β4 ( TB4 )是一種 43 個氨基酸的多肽,是細胞遷移和分化的重要介質,還促進血管生成和傷口癒合。
Philp 等人報導,TB4 刺激正常大鼠和小鼠的毛髮生長。小鼠皮膚中的毛囊角質形成細胞的特定子,集在毛髮生長周期中,以高度協調的方式表達 TB4。這些角質形成細胞起源於毛囊隆起區域,這是皮膚幹細胞的利基市場。分離到與隆起的干細胞密切相關(即使不完全相同)的鼠弧菌毛囊克隆性角質形成細胞,並在納摩爾濃度的 TB4 存在下,增加其遷移和分化。
TB4 增加了細胞外基質降解酶基質金屬蛋白酶-2 的表達和分泌。因此,TB4 加速了頭髮的生長,部分原因是它對毛囊週期活躍期中的關鍵事件,產生了影響,包括促進乾細胞及其直接後代,向毛囊底部遷移、分化、以及細胞外基質重塑。
最近的一份報告確定轉化生長因子-β家族成員激活素、是皮膚形態發生、修復、和頭髮生長的有效調節劑。然而,過分表達分泌的激活素拮抗劑卵泡抑素,令小鼠的頭髮生長減少。
在角質形成細胞中,表達顯性負激活素受體 IB 突變體 ( dnActRIB )的小鼠,具有未改變的成年皮膚結構,但在產後卵形毛囊形態發生,和毛囊的第一個致癌基因-端粒轉化中,觀察到延遲。影響組織培養或小鼠皮膚中乙酰肝素酶,TB4 或激活素表達水平的 LLLT 的表達,但這些分子是進一步研究以解釋 LLLT 誘導毛髮生長的良好候選物。
參考醫學文獻
已有餘千的出版刊物,報導激光或低能量激光能有效地增加細胞的存活,增殖和功能。臨床對照試驗顯示,激光能夠刺激和保存受雄激素性脫髮及其他脫髮症影响毛囊。
[1] E. Mester, B. Szende and P. Gartner, The effect of laser beams on the growth of hair in mice, Radiobiol Radiother (Berl) 9 (1968) 621-6.
[2] R. Roelandts, The history of phototherapy: something new under the sun?, J Am Acad Dermatol 46 (2002) 926-30.
[3] A.N. Pereira, P. Eduardo Cde, E. Matson and M.M. Marques, Effect of low-power laser irradiation on cell growth and procollagen synthesis of cultured fibroblasts, Lasers Surg Med 31 (2002) 263-7.
[4] J.S. Kana, G. Hutschenreiter, D. Haina and W. Waidelich, Effect of low-power density laser radiation on healing of open skin wounds in rats, Arch Surg 116 (1981) 293-6.
[5] A.P. Sommer, A.L. Pinheiro, A.R. Mester, R.P. Franke and H.T. Whelan, Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system, J Clin Laser Med Surg 19 (2001) 29-33.
[6] J.C. Sutherland, Biological effects of polychromatic light, Photochem Photobiol 76 (2002) 164-70.
[7] T. Karu, Laser biostimulation: a photobiological phenomenon, J Photochem Photobiol B 3 (1989) 638-40.
[8] T.I. Karu and N.I. Afanas’eva, Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light, Dokl Akad Nauk 342 (1995) 693-5.
[9] R.A. Capaldi, F. Malatesta and V.M. Darley-Usmar, Structure of cytochrome c oxidase, Biochim Biophys Acta 726 (1983) 135-48.
[10] I. Szundi, G.L. Liao and O. Einarsdottir, Near-infrared time-resolved optical absorption studies of the reaction of fully reduced cytochrome c oxidase with dioxygen, Biochemistry 40 (2001) 2332-9.
[11] T.I. Karu and S.F. Kolyakov, Exact action spectra for cellular responses relevant to phototherapy, Photomed Laser Surg 23 (2005) 355-61.
[12] D. Pastore, M. Greco and S. Passarella, Specific helium-neon laser sensitivity of the purified cytochrome c oxidase, Int J Radiat Biol 76 (2000) 863-70.
[13] V.G. Artyukhov, O.V. Basharina, A.A. Pantak and L.S. Sveklo, Effect of helium-neon laser on activity and optical properties of catalase, Bull Exp Biol Med 129 (2000) 537-40.
[14] W. Yu, J.O. Naim, M. McGowan, K. Ippolito and R.J. Lanzafame, Photomodulation of oxidative metabolism and electron chain enzymes in rat liver mitochondria, Photochem Photobiol 66 (1997) 866-71.
[15] S. Passarella, He-Ne laser irradiation of isolated mitochondria, J Photochem Photobiol B 3 (1989) 642-3.
[16] S.J. Ehrreich and R.F. Furchgott, Relaxation of mammalian smooth muscles by visible and ultraviolet radiation, Nature 218 (1968) 682-4.
[17] H. Chaudhry, M. Lynch, K. Schomacker, R. Birngruber, K. Gregory and I. Kochevar, Relaxation of vascular smooth muscle induced by low-power laser radiation, Photochem Photobiol 58 (1993) 661-9.
[18] R. Mittermayr, A. Osipov, C. Piskernik, S. Haindl, P. Dungel, C. Weber, Y.A. Vladimirov, H. Redl and A.V. Kozlov, Blue laser light increases perfusion of a skin flap via release of nitric oxide from hemoglobin, Mol Med 13 (2007) 22-9.
[19] L. Vladimirov, A., G.I. Klebanov, G.G. Borisenko and A.N. Osipov, Molecular and cellular mechanisms of the low intensity laser radiation effect, Biofizika 49 (2004) 339-50.
[20] Y.A. Vladimirov, A.N. Osipov and G.I. Klebanov, Photobiological principles of therapeutic applications of laser radiation, Biochemistry (Mosc) 69 (2004) 81-90.
[21] G.G. Borisenko, A.N. Osipov, K.D. Kazarinov and A. Vladimirov Yu, Photochemical reactions of nitrosyl hemoglobin during exposure to low-power laser irradiation, Biochemistry (Mosc) 62 (1997) 661-6.
[22] B. Beltran, A. Mathur, M.R. Duchen, J.D. Erusalimsky and S. Moncada, The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death, Proc Natl Acad Sci U S A 97 (2000) 14602-7.
[23] G.C. Brown, Regulation of mitochondrial respiration by nitric oxide inhibition of cytochrome c oxidase, Biochim Biophys Acta 1504 (2001) 46-57.
[24] N. Lane, Cell biology: power games, Nature 443 (2006) 901-3.
[25] G.C. Brown and V. Borutaite, Nitric oxide inhibition of mitochondrial respiration and its role in cell death, Free Radic Biol Med 33 (2002) 1440-50.
[26] P. Ghafourifar and E. Cadenas, Mitochondrial nitric oxide synthase, Trends Pharmacol Sci 26 (2005) 190-5.
[27] E. Clementi, G.C. Brown, N. Foxwell and S. Moncada, On the mechanism by which vascular endothelial cells regulate their oxygen consumption, Proc Natl Acad Sci U S A 96 (1999) 1559-62.
[28] F. Antunes, A. Boveris and E. Cadenas, On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide, Proc Natl Acad Sci U S A 101 (2004) 16774-9.
[29] T.I. Karu, L.V. Pyatibrat and N.I. Afanasyeva, Cellular effects of low power laser therapy can be mediated by nitric oxide, Lasers Surg Med 36 (2005) 307-14.
[30] V. Borutaite, A. Budriunaite and G.C. Brown, Reversal of nitric oxide-, peroxynitrite- and S-nitrosothiol-induced inhibition of mitochondrial respiration or complex I activity by light and thiols, Biochim Biophys Acta 1459 (2000) 405-12.
[31] G.A. Guzzardella, M. Fini, P. Torricelli, G. Giavaresi and R. Giardino, Laser stimulation on bone defect healing: an in vitro study, Lasers Med Sci 17 (2002) 216-20.
[32] H. Tuby, L. Maltz and U. Oron, Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis, Lasers Surg Med 38 (2006) 682-8.
[33] Y. Moriyama, E.H. Moriyama, K. Blackmore, M.K. Akens and L. Lilge, In vivo study of the inflammatory modulating effects of low-level laser therapy on iNOS expression using bioluminescence imaging, Photochem Photobiol 81 (2005) 1351-5.
[34] M.C. Leung, S.C. Lo, F.K. Siu and K.F. So, Treatment of experimentally induced transient cerebral ischemia with low energy laser inhibits nitric oxide synthase activity and up-regulates the expression of transforming growth factor-beta 1, Lasers Surg Med 31 (2002) 283-8.
[35] H. Friedmann, R. Lubart, I. Laulicht and S. Rochkind, A possible explanation of laser-induced stimulation and damage of cell cultures, J Photochem Photobiol B 11 (1991) 87-91.
[36] M. Eichler, R. Lavi, A. Shainberg and R. Lubart, Flavins are source of visible-light-induced free radical formation in cells, Lasers Surg Med 37 (2005) 314-9.
[37] K. Plaetzer, T. Kiesslich, B. Krammer and P. Hammerl, Characterization of the cell death modes and the associated changes in cellular energy supply in response to AlPcS4-PDT, Photochem Photobiol Sci 1 (2002) 172-7.
[38] R. Lubart, M. Eichler, R. Lavi, H. Friedman and A. Shainberg, Low-energy laser irradiation promotes cellular redox activity, Photomed Laser Surg 23 (2005) 3-9.
[39] R. Duan, T.C. Liu, Y. Li, H. Guo and L.B. Yao, Signal transduction pathways involved in low intensity He-Ne laser-induced respiratory burst in bovine neutrophils: a potential mechanism of low intensity laser biostimulation, Lasers Surg Med 29 (2001) 174-8.
[40] F.Q. Schafer and G.R. Buettner, Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple, Free Radic Biol Med 30 (2001) 1191-212.
[41] H. Liu, R. Colavitti, Rovira, II and T. Finkel, Redox-dependent transcriptional regulation, Circ Res 97 (2005) 967-74.
[42] M. Yang, N.B. Nazhat, X. Jiang, S.M. Kelsey, D.R. Blake, A.C. Newland and C.J. Morris, Adriamycin stimulates proliferation of human lymphoblastic leukaemic cells via a mechanism of hydrogen peroxide (H2O2) production, Br J Haematol 95 (1996) 339-44.
[43] W.G. Kirlin, J. Cai, S.A. Thompson, D. Diaz, T.J. Kavanagh and D.P. Jones, Glutathione redox potential in response to differentiation and enzyme inducers, Free Radic Biol Med 27 (1999) 1208-18.
[44] S. Alaluf, H. Muir-Howie, H.L. Hu, A. Evans and M.R. Green, Atmospheric oxygen accelerates the induction of a post-mitotic phenotype in human dermal fibroblasts: the key protective role of glutathione, Differentiation 66 (2000) 147-55.
[45] T. Karu, Primary and secondary mechanisms of action of visible to near-IR radiation on cells, J Photochem Photobiol B 49 (1999) 1-17.
[46] O. Tiphlova and T. Karu, Action of low-intensity laser radiation on Escherichia coli, Crit Rev Biomed Eng 18 (1991) 387-412.
[47] T.I. Karu, L.V. Pyatibrat, G.S. Kalendo and R.O. Esenaliev, Effects of monochromatic low-intensity light and laser irradiation on adhesion of HeLa cells in vitro, Lasers Surg Med 18 (1996) 171-7.
[48] P. Moore, T.D. Ridgway, R.G. Higbee, E.W. Howard and M.D. Lucroy, Effect of wavelength on low-intensity laser irradiation-stimulated cell proliferation in vitro, Lasers Surg Med 36 (2005) 8-12.
[49] D. Hawkins and H. Abrahamse, Biological effects of helium-neon laser irradiation on normal and wounded human skin fibroblasts, Photomed Laser Surg 23 (2005) 251-9.
[50] H.S. Yu, C.S. Wu, C.L. Yu, Y.H. Kao and M.H. Chiou, Helium-neon laser irradiation stimulates migration and proliferation in melanocytes and induces repigmentation in segmental-type vitiligo, J Invest Dermatol 120 (2003) 56-64.
[51] S. Young, P. Bolton, M. Dyson, W. Harvey and C. Diamantopoulos, Macrophage responsiveness to light therapy, Lasers Surg Med 9 (1989) 497-505.
[52] Y. Fujimaki, T. Shimoyama, Q. Liu, T. Umeda, S. Nakaji and K. Sugawara, Low-level laser irradiation attenuates production of reactive oxygen species by human neutrophils, J Clin Laser Med Surg 21 (2003) 165-70.
[53] Y.S. Chen, S.F. Hsu, C.W. Chiu, J.G. Lin, C.T. Chen and C.H. Yao, Effect of low-power pulsed laser on peripheral nerve regeneration in rats, Microsurgery 25 (2005) 83-9.
[54] M. Miloro, L.E. Halkias, S. Mallery, S. Travers and R.G. Rashid, Low-level laser effect on neural regeneration in Gore-Tex tubes, Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93 (2002) 27-34.
[55] P. Balaban, R. Esenaliev, T. Karu, E. Kutomkina, V. Letokhov, A. Oraevsky and N. Ovcharenko, He-Ne laser irradiation of single identified neurons, Lasers Surg Med 12 (1992) 329-37.
[56] K.R. Byrnes, R.W. Waynant, I.K. Ilev, X. Wu, L. Barna, K. Smith, R. Heckert, H. Gerst and J.J. Anders, Light promotes regeneration and functional recovery and alters the immune response after spinal cord injury, Lasers Surg Med 36 (2005) 171-85.
[57] S.O. el Sayed and M. Dyson, Effect of laser pulse repetition rate and pulse duration on mast cell number and degranulation, Lasers Surg Med 19 (1996) 433-7.
[58] R.A. Lopes-Martins, R. Albertini, P.S. Martins, J.M. Bjordal and H.C. Faria Neto, Spontaneous effects of low-level laser therapy (650 nm) in acute inflammatory mouse pleurisy induced by Carrageenan, Photomed Laser Surg 23 (2005) 377-81.
[59] A.D. Agaiby, L.R. Ghali, R. Wilson and M. Dyson, Laser modulation of angiogenic factor production by T-lymphocytes, Lasers Surg Med 26 (2000) 357-63.
[60] S. Passarella, E. Casamassima, S. Molinari, D. Pastore, E. Quagliariello, I.M. Catalano and A. Cingolani, Increase of proton electrochemical potential and ATP synthesis in rat liver mitochondria irradiated in vitro by helium-neon laser, FEBS Lett 175 (1984) 95-9.
[61] M. Greco, G. Guida, E. Perlino, E. Marra and E. Quagliariello, Increase in RNA and protein synthesis by mitochondria irradiated with helium-neon laser, Biochem Biophys Res Commun 163 (1989) 1428-34.
[62] D. Pastore, M. Greco, V.A. Petragallo and S. Passarella, Increase in H+/e- ratio of the cytochrome c oxidase reaction in mitochondria irradiated with helium-neon laser, Biochem Mol Biol Int 34 (1994) 817-26.
[63] Y. Zhang, S. Song, C.C. Fong, C.H. Tsang, Z. Yang and M. Yang, cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light, J Invest Dermatol 120 (2003) 849-57.
[64] R.F. Lyons, R.P. Abergel, R.A. White, R.M. Dwyer, J.C. Castel and J. Uitto, Biostimulation of wound healing in vivo by a helium-neon laser, Ann Plast Surg 18 (1987) 47-50.
[65] H.S. Yu, K.L. Chang, C.L. Yu, J.W. Chen and G.S. Chen, Low-energy helium-neon laser irradiation stimulates interleukin-1 alpha and interleukin-8 release from cultured human keratinocytes, J Invest Dermatol 107 (1996) 593-6.
[66] V.K. Poon, L. Huang and A. Burd, Biostimulation of dermal fibroblast by sublethal Q-switched Nd:YAG 532 nm laser: collagen remodeling and pigmentation, J Photochem Photobiol B 81 (2005) 1-8.
[67] N. Kipshidze, V. Nikolaychik, M.H. Keelan, L.R. Shankar, A. Khanna, R. Kornowski, M. Leon and J. Moses, Low-power helium: neon laser irradiation enhances production of vascular endothelial growth factor and promotes growth of endothelial cells in vitro, Lasers Surg Med 28 (2001) 355-64.
[68] A. Khanna, L.R. Shankar, M.H. Keelan, R. Kornowski, M. Leon, J. Moses and N. Kipshidze, Augmentation of the expression of proangiogenic genes in cardiomyocytes with low dose laser irradiation in vitro, Cardiovasc Radiat Med 1 (1999) 265-9.
[69] A.R. Medrado, L.S. Pugliese, S.R. Reis and Z.A. Andrade, Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts, Lasers Surg Med 32 (2003) 239-44.
[70] E.J. Neiburger, Rapid healing of gingival incisions by the helium-neon diode laser, J Mass Dent Soc 48 (1999) 8-13, 40.
[71] J.T. Eells, M.M. Henry, P. Summerfelt, M.T. Wong-Riley, E.V. Buchmann, M. Kane, N.T. Whelan and H.T. Whelan, Therapeutic photobiomodulation for methanol-induced retinal toxicity, Proc Natl Acad Sci U S A 100 (2003) 3439-44.
[72] M.T. Wong-Riley, H.L. Liang, J.T. Eells, B. Chance, M.M. Henry, E. Buchmann, M. Kane and H.T. Whelan, Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase, J Biol Chem 280 (2005) 4761-71.
[73] D. Gigo-Benato, S. Geuna and S. Rochkind, Phototherapy for enhancing peripheral nerve repair: a review of the literature, Muscle Nerve 31 (2005) 694-701.
[74] J.J. Anders, S. Geuna and S. Rochkind, Phototherapy promotes regeneration and functional recovery of injured peripheral nerve, Neurol Res 26 (2004) 233-9.
[75] J.J. Anders, R.C. Borke, S.K. Woolery and W.P. Van de Merwe, Low power laser irradiation alters the rate of regeneration of the rat facial nerve, Lasers Surg Med 13 (1993) 72-82.
[76] K. Branco and M.A. Naeser, Carpal tunnel syndrome: clinical outcome after low-level laser acupuncture, microamps transcutaneous electrical nerve stimulation, and other alternative therapies–an open protocol study, J Altern Complement Med 5 (1999) 5-26.
[77] J. Irvine, S.L. Chong, N. Amirjani and K.M. Chan, Double-blind randomized controlled trial of low-level laser therapy in carpal tunnel syndrome, Muscle Nerve 30 (2004) 182-7.
[78] M.I. Weintraub, Noninvasive laser neurolysis in carpal tunnel syndrome, Muscle Nerve 20 (1997) 1029-31.
[79] Y. Ueda and N. Shimizu, Pulse irradiation of low-power laser stimulates bone nodule formation, J Oral Sci 43 (2001) 55-60.
[80] Y. Ueda and N. Shimizu, Effects of pulse frequency of low-level laser therapy (LLLT) on bone nodule formation in rat calvarial cells, J Clin Laser Med Surg 21 (2003) 271-7.
[81] M.S. Ribeiro, F. Da Silva Dde, C.E. De Araujo, S.F. De Oliveira, C.M. Pelegrini, T.M. Zorn and D.M. Zezell, Effects of low-intensity polarized visible laser radiation on skin burns: a light microscopy study, J Clin Laser Med Surg 22 (2004) 59-66.
[82] T. Moshkovska and J. Mayberry, It is time to test low level laser therapy in Great Britain, Postgrad Med J 81 (2005) 436-41.
[83] L.A. Santana-Blank, E. Rodriguez-Santana and K.E. Santana-Rodriguez, Photo-infrared pulsed bio-modulation (PIPBM): a novel mechanism for the enhancement of physiologically reparative responses, Photomed Laser Surg 23 (2005) 416-24.
[84] M.L. Kripke, Ultraviolet radiation and immunology: something new under the sun–presidential address, Cancer Res 54 (1994) 6102-5.
[85] P. Whittaker, Laser acupuncture: past, present, and future, Lasers Med Sci 19 (2004) 69-80.
脫髮 & Blog 首頁
